Evaluation of the Institute of Cancer Research (ICR)
Report of the ICR Evaluation Panel
March 2017
At the Canadian Institutes of Health Research (CIHR), we know that research has the power to change lives. As Canada's health research investment agency, we collaborate with partners and researchers to support the discoveries and innovations that improve our health and strengthen our health care system.
Canadian Institutes of Health Research
160 Elgin Street, 9th Floor
Address Locator 4809A
Ottawa, Ontario K1A 0W9
This publication was produced by the Canadian Institutes of Health Research. The views expressed herein do not necessarily reflect those of the Canadian Institutes of Health Research.
ICR Evaluation Panel
Chair
Ann Chambers
Professor, Department of Oncology
Schulich School of Medicine and Dentistry, University of Western Ontario
Panel Members
Pamela Hoodless
Professor, Medical Genetics, University of British Columbia
Roger Deeley
Vice-Dean, Health Sciences Research, Queen's University
Ken Kao
Professor, Division of Biomedical Science, Faculty of Medicine, Memorial University
Acknowledgements
Thanks to all participants in this evaluation –survey respondents, interview and case study participants – and to Goss Gilroy Inc., Circum Network Inc., for data collection and analysis. Additional thanks to the ICR CIHR Evaluation Team: Michael Goodyer, Carmen, Constantinescu, David Peckham, Abigail Forson, Christopher Manuel and Carole Chow. And special thanks to the CIHR-ICR Scientific Director Dr. Stephen Robbins and Dr. Rachel Syme Assistant Director, ICR.
Cover art: Let the Skyworld Shake (2016) by Lisa Boivin
For more information and to obtain copies, please contact: Evaluation@cihr-irsc.gc.ca
Table of Contents
- I. Executive Summary
- II. Overview of the Evaluation
- III. Observations and Recommendations
- IV. Evaluation Key Findings
- V. References
- VI. Appendices
I. Executive Summary
The evaluation of the Institute of Cancer Research (ICR) was undertaken by the Canadian Institutes of Health Research (CIHR) as part of the review of the mandate and performance of CIHR Institutes by CIHR’s Governing Council (GC) outlined in the CIHR Act. The evaluation assessed the relevance and performance of ICR to inform GC decisions regarding the role and functioning of the Institute and renewal of the current Scientific Director. The evaluation was conducted by the CIHR Evaluation Unit and a team of external evaluation professionals and overseen by a panel of experts in ICR’s mandate areas who reviewed and interpreted the findings and made the final recommendations. The key recommendations of the Panel are summarized below by the three GC decision items.
1. Should the ICR be amended, merged or terminated?
The human and economic burden of cancer is significant in Canada and expected to increase as the population ages. The last 10 years have seen promising developments in the scientific landscape which have been supported through funding from CIHR, as well as other organizations. The Panel believes it is crucial that CIHR continue to have a strong voice in guiding the national cancer research strategy and that the ICR by virtue of its consistently strong leadership, credible presence and knowledge of the field is well-placed to play this role. The Panel recommends that the ICR not be amended, merged or terminated.
2. Should the ICR’s mandate be changed?
While the mandate of the ICR is broad, the strategic priorities provide focus to the work of the Institute within its limited funding envelope. The Panel feels that the mandate is appropriate and supports the ICR’s strategic priorities. While future priorities specifically target areas that do not appear to encompass the research interests of many in the community, especially in the area of basic biomedical research, several initiatives from previous strategic priorities focused in this area are ongoing. The Panel recommends that the current mandate remain unchanged, to encompass the spectrum of research necessary to address the burden of cancer in Canada.
3. Should the ICR Scientific Director be renewed?
The ICR has benefitted from strong leadership since its inception and the Panel commends the leadership provided by the current Scientific Director during a challenging period of transition at CIHR. The Scientific Director has demonstrated strong skills in engaging various sectors of the cancer research community and is well-regarded by both researchers and stakeholders. During his tenure, the ICR has been successful in collaborating with a variety of organizations and has leveraged significant funding to support the objectives of the Institute. The Panel recommends that the current ICR Scientific Director be renewed.
II. Overview of the Evaluation
Institute of Cancer Research
As one of the 13 CIHR Institutes, the Institute of Cancer Research (ICR) has a mandate to support research that reduces the burden of cancer on individuals and families through prevention strategies, screening, diagnosis, effective treatments, psycho-social support systems, and palliation. The ICR’s mission is to foster research based on internationally accepted standards of excellence, which bears on preventing and treating cancer, and improving the health and quality of life of cancer patients and survivors. The ICR’s vision is to be recognized as a dynamic research organization that:
- Takes a lead role in the development of a national strategic cancer research agenda.
- Interacts with other agencies – federal, provincial, and non-governmental organizations – to fund research that supports cancer control priorities as established through national consultation.
- Creates and maintains a robust cancer research environment in Canada that attracts and sustains excellent young researchers, established world-class investigators and research teams.
- Improves the health of Canadians by supporting cross-cutting research initiatives that lead to enhanced cancer prevention, diagnosis, and treatment.
Evaluation Objectives
The evaluation of the ICR was conducted by CIHR as part of a rolling review of the mandate and performance of CIHR Institutes. The aims of the evaluation are to provide GC with valid and reliable findings to inform decisions regarding:
- Should the ICR be amended, merged or terminated?
- Should the ICR’s mandate be changed?
- Should the ICR Scientific Director be renewed?
The evaluation was overseen by the ICR Evaluation Panel (hereafter referred to as the Panel) comprised of experts in the ICR’s mandate areas and conducted by the CIHR Evaluation Unit and external evaluation professionals. The names and affiliations of the Panel members are listed in Appendix 1. The evaluation examined the period 2000-2016, with a specific focus on the period under the leadership of the current Scientific Director (SD) Dr. Stephen RobbinsFootnote 1. The evaluation of ICR was informed by multiple lines of evidence, including: the review of documents and data, interviews with ICR and CIHR staff and partners, surveys of researchers and stakeholders, and an impact study on ICR related research within and beyond academia. The methods and data sources are outlined in Appendix 2 and key figures presented in Appendix 3. While each line of evidence has limitations, there is convergence among them so as to produce key findings. Overall, we are reasonably confident that the results presented provide an accurate portrait of the ICR’s relevance and performance.
III. Observations and Recommendations
Should the ICR be Amended, Merged or Terminated?
Context
Globally, over 14 million individuals are diagnosed with cancer each year. Within the next two decades, this number is expected to increase to 22 million. Without significant improvements, by the year 2030, more than 17 million people are expected to succumb to the disease worldwide.Footnote 2 In Canada, in 2011, 30% of deaths were related to cancer.Footnote 3 Overall, it is estimated that about 2 in 5 Canadians will develop cancer in their lifetimes and 1 in 4 will die of the disease.Footnote 4
The number of new cancer cases – approximately 202,400 new cases in 2016 in Canada – has been increasing steadily since 1987 primarily due to an aging population and, to a lesser extent, population growth and changes in the risk of developing cancer.Footnote 5 Further, cancer diagnoses are projected to change with increases in thyroid cancer, melanoma and uterine cancers. Lung cancer is projected to remain the top cancer killer throughout this time period; however, pancreas and liver cancers are projected to surpass breast, prostate, and colorectal cancers to become the second and third leading causes of cancer-related death, respectively.Footnote 6 Figure A in Appendix 3 depicts the proportion of deaths due to cancer in Canada in 2011.
The most recent estimates available indicate that, cancer is the 7th most costly illness or injury in Canada accounting for $4.4B in economic costs. This includes $3.8B in direct healthcare costs (includes hospital, drug and physician costs) and $586M in indirect costs from lost productivity due to illness or premature death.Footnote 7
Scientific and Funding Landscape
The cancer research scientific landscape has evolved in the last 10 years. Novel targeted cancer therapies and companion diagnostics harnessing basic discovery research continue to dominate the treatment horizon and there have been significant advancements in genomics, personalized medicine and emergence of promising new research in areas such as immunotherapy. Due to improvements in cancer detection and treatment arising from research, the mortality rate of many cancers has decreased.Footnote 8
With respect to cancer research funding, the environment is exceptionally complex compared to many major diseases with a multiplicity of funders in the cancer research ecosystem. This complexity is managed to some degree by the Canadian Cancer Research Alliance (CCRA) which brings together 35 cancer research funding organizations and is co-chaired by the current ICR SD, Dr. Robbins, who was elected from among the organizational representatives.Footnote 9
Recent data reported by the CCRAFootnote 10 indicates that in 2013 there was a total of $498.2M in contributions to cancer research by CCRA-affiliated Canadian organizations/programsFootnote 11. While significant, this was the lowest annual amount since 2008.Footnote 12
CIHR is the largest overall funder of cancer research in Canada; in 2013, CIHR invested $141.4M in cancer research, representing about 15% of total CIHR grants and awards. Cancer research capacity in Canada is significant; each year, there are 2000+ CIHR-funded cancer researchers in Canada, 200+ directly supported trainees and 1,300+ indirectly supported trainees in this mandate area. Overall, however, the number of CIHR grants and awards and amount of research funding in the ICR mandate area has decreased in the last two years. Further, recent changes at CIHR as well as reductions in funding from several voluntary cancer organizations have put increasing pressure on Canadian cancer researchers.
Since 2007-08, the annual research budget for the ICR has been $8.6M, but was reduced in 2014-15 to $4.3M, representing less than 1% of total annual investments in cancer in Canada and about 3% of total CIHR investments in the ICR mandate area. Figure B in Apendix 3 shows the percentage of ICR RC investment out of CIHR investment in ICR mandate over the years. The other $4.3M is invested in CIHR’s Roadmap Accelerator Fund (RAF), along with the same amount from each of the other institutes, to support multi-institute and multi-disciplinary initiatives aligned with CIHR research priorities. Although ICR does not have direct control over the RAF, but can promote initiatives aligned with the ICR mandate.
Panel Observations
Achieving its mandate
The evaluation indicates that the ICR is an important and relevant mechanism for the advancement of cancer-related research. The Panel notes the Institute’s successes and achievements in direct support of internationally recognized high caliber research; from 2000-01 to 2014-15, ICR disbursed $103M in research grants in ICR’s mandate area and since 2012-13, a total of 13 funding opportunities were launched. The Institute has emphasized novel research through Innovation Grants that addressed priority areas such as high fatality cancers. This view is confirmed by researchers: 54% of funded researchers agreed that the ICR funding supported innovative ideas in their own research to a great extent.
To help achieve its mandate, the Panel notes that the ICR has made strategic investments in capacity building, with an emphasis on New Investigators. Annual New Investigator meetings contribute to the professional development of junior faculty working in cancer research and the biennial Canadian Cancer Research Conference includes sessions targeted to trainees. Overall, 60% of researchers said their ICR funding supported training of researchers or practitioners to a great extent.
ICR is recognized for its knowledge translation activities to improve health services. Current efforts such as Knowledge to Action Grants, Best Brain Exchanges and Café Scientifiques will be enhanced in the coming years with the funding of projects under the Institute’s health economics/health services priority. A direct link between ICR-funded research and improvements to health services and health of Canadians is difficult to establish. However, a new study by ICR is mapping the impact of early investments in research on palliative cancer care on health policy (impact on assisted dying bill), knowledge translation (publications), application (patents), and capacity building in the field. Impact study data from the evaluation illustrate that there has been a steady increase (from 2008 to 2015) in the overall number of ICR mandate related publications published each year.
Critically important to the ICR’s achievements has been partnerships with other Institutes. The ICR has made multiple contributions to CIHR Signature Initiatives, and is the co-lead for two initiatives. With the Institute of Genetics and Institute of Neurosciences, Mental Health and Addiction, ICR co-leads the Canadian Epigenetics, Environment and Health Research Consortium – a CIHR Signature Initiative aimed at ensuring that Canada plays a leadership role in the field of epigenetics. Also with the Institute of Genetics, ICR is co-lead for the Personalized Medicine Signature Initiative, which supports supporting translational research for prevention, diagnostic, and treatment of cancer.
ICR has also partnered and leveraged significant funding through collaborations with other organizations in the cancer research field. Notably, ICR is a key partner in funding a multi-disciplinary, multi-site Dream Team in the area of cancer stem cells with Genome Canada, Stand Up To Cancer and others. In total, ICR leveraged $80M in contributions from 52 partners between 2000-01 and 2014-15 and the leveraging ratio has increased during the tenure of the current Scientific Director.
Figure C in Appendix 3 shows the partners’ contributions to ICR priorities, ICR Responsibility Centre (RC) investments and leverage ratio of partnership from 2001-02 to 2015-16.
Position within the Canadian Cancer Research Environment
The scientific and funding landscape in the cancer research area is complex. There is an ongoing need for a national voice for the cancer research community. The Panel notes that the National Cancer Institute of Canada (NCIC) was previously viewed as contributing to this role. Presently, the ICR is filling this important role as national leader and voice for the cancer community. The Panel feels strongly that CIHR, through ICR, must fill this national mandate. ICR has arguably begun to fill that void, particularly through the activities of its Scientific Directors and the CCRA. As ICR is one of the institutes of the federal health research funding agency, the Panel feels that the ICR is well-placed to solidify its role as the national voice and leader in the cancer research area. ICR is perceived as being the primary funder of cancer research in Canada. This is not only due in part to limited awareness among the cancer research community of the distinction between investigator-initiated (open) and priority-driven (strategic) funding but also to the success of the ICR Scientific Director and the efforts of the Institute to connect with and advocate for the community in leveraging funding for strategic research priorities.
The Panel recognizes the vital and much needed role of the ICR within CIHR given the burden of cancer and the important role of CIHR as a funder of cancer research. This role is even more important in an era of decreasing funding which is leading to concerns in the community about the vulnerability of cancer research funding. The Panel feels it essential that the ICR continue to be an effective voice for cancer research across Canada.
Recommendations
Should the ICR be amended, merged or terminated?
Recommendation 1: The Panel strongly recommends that the ICR should not be amended, merged or terminated.
Recommendation 2: The Panel strongly recommends that the ICR continue as a separate institute within the CIHR.
Should ICR’s mandate be changed?
Context
The ICR mandate addresses the cancer continuum, including prevention strategies, screening, diagnosis, effective treatments, psycho-social support systems, and palliation. With its limited funding envelope, the ICR has consulted with the community to select three current strategic priorities to focus its efforts in a few key areas where it can be impactful – high fatality cancers, health economics/health services and redressing risk factor disparities.
To address High Fatality Cancers, the ICR recently initiated two Innovation Grant competitions to provide one-year funding, and ICR contributes funding to CTRnet (Canadian Tumour Repository Network) and Stand Up to Cancer Stem Cell Dream Team (partner with CSCC and Genome Canada). Health services/health economics initiatives include Innovation Grants and grants focused on developing Partnerships for Health System Improvement in Cancer Control. To redress risk factor disparities, ICR has two funding initiatives, the Prevention grant for vulnerable populations and the Catalyst Grant for Indigenous Approaches to Wellness, partnered with the Institute of Aboriginal Peoples' Health (IAPH).In addition to funding opportunities to support strategic priorities, the Institute seeks other ways to influence the cancer research ecosystem and further its mandate such as through investments in new investigator meetings, leadership within the CCRA and knowledge mobilization.
ICR also plays a role in three ongoing Signature Initiatives: Personalized Medicine, Community Based Primary Health Care and the Canadian Epigenetics, Environment and Health Research Consortium.
Panel Observations
The Panel recognizes that the ICR’s broad mandate is important for the Institute to ensure inclusiveness of all sectors of the cancer research community. The strategic priorities that the Institute has selected to frame and focus its efforts within its broad mandate are supported by the Panel. The priorities have been judiciously identified and represent gaps or opportunities where ICR can make a difference. The Panel is concerned, however, that while the ICR IAB was previously involved in the selection of the priorities, with the loss of institute-specific IABs (see below), their implementation must now occur in its absence and cannot therefore benefit from its expertise.
The Panel feels that CIHR funds and funding mechanisms are limited to support the ICR in fulfilling its mandate. The reduction in funds directly available to the Institute in 2015-16 and the loss of CIHR funding programs such as the Strategic Training Initiative in Health Research (STIHRs) impede the Institute’s achievement of objectives. Moving forward, partnerships will be critically important for the Institute to advance its objectives. Considering the Institute’s strategic priorities, the Panel sees important opportunities for collaboration between ICR and other institutes in shared mandate areas (e.g., IHSPR and IAPH).
The panel commends the ICR for reaching out to include the community in themes 3 and 4. However, the current strategic priorities align with the interests of a small sector of the research community and some researchers feel the mandate could be broadened to more explicitly include basic research.
Recommendations
The Panel agrees that the ICR’s mandate is appropriate, but given the Institute’s resources, it may be too broad in scope to be achieved with ICR resources alone.
Should the ICR’s mandate be changed?
Recommendation 3: The Panel recommends that the ICR continue with its current mandate.
Future Considerations
- The Panel suggests that ICR continue to partner with other institutes with related mandates, serving to strengthen and improve capacity while reducing duplication of efforts.
- The Panel suggests that the ICR continue to use approaches that do not require considerable financial support to achieve its broad mandate (i.e., SD working through CCRA, SD networking with the research community Canada-wide).
- The Panel suggests that the ICR make formal requests to GC, perhaps in conjunction with other CIHR institutes, to increase institutes’ budgets.
- The Panel suggests that the ICR continue to impress on CIHR the essential role of the ICR as the central body responsible for cancer research nationally.
Should the current ICR Scientific Director be renewed?
Context
The current ICR Scientific Director, Dr. Stephen Robbins, has held the position since 2013. He also is Co-Chair of the CCRA.
During the first term of the SD, several changes at CIHR were implemented with the goal of enhancing collaboration and transversal thinking across Institutes. First, the IABs for each Institute were disbanded in favor of five cross-cutting thematic IABs that can advise any Institute. Second, Ottawa-based Institute Staff (OBIS) were restructured from providing services to one Institute to providing specific management expertise across Institutes in areas required to support the activities, for example initiating a funding opportunity.
Panel Observations
The ICR Scientific Director is highly regarded by the cancer research community as a leading source of information in the field, as well as for being an inclusive and active spokesperson and advocate for cancer research. The evaluation found that under the leadership of the current ICR Scientific Director, the Institute has identified and leveraged strategic research investments in high impact areas. The Panel also notes his contributions as an effective member of CIHR management team and collaborator with other Institutes and the broader cancer research funding community.
The ICR has benefited from three exceptional Scientific Directors since its inception. Like previous Scientific Directors, Dr. Robbins has been effective in building partnerships and notably has reached out to and interacts with communities in all the health research themes. The Panel particularly commends Dr. Robbins’ strong leadership during a challenging period of transition at CIHR which has seen the budget of the ICR reduced and the elimination of the IAB. A change in leadership at this time could, in fact, be detrimental to the Institute. Rather, the Panel suggests that the role of the ICR Scientific Director be reinforced as the lead for cancer research in Canada. The Panel cautions that in the absence of Institute specific IABs, the responsibility to seek advice falls to the Scientific Directors which could place them in a position of perceived or implicit bias.The Panel feels that there is a need for Ottawa-based Institute staff (OBIS) with cancer-specific content expertise since advice to the SD and Institute is now completed on an ad hoc basis due to a reduction of resources. The Panel notes that the SD is doing an admirable job at seeking out advice from the broad cancer community, but feels that CIHR should be assisting him in this role.
In addition to its annual research budget, the ICR receives $1M annually through the Institute Support Grant (ISG) for operating and development expenditures. Over the period from 2009-10 to 2015-16, the ICR typically spent its full annual allotment of $1M; however, the carry forward of the unspent balance was approximately 50-60% of the total funds available under the ISG in a given year (i.e., annual allotment plus carry forward).
The panel also notes and commends the efforts of the SD to link with Lisa Boivin, artist and member of the Deninu Kue First Nation in Northwest Territories, to describe the cancer journey for Indigenous populations through her art. It is hoped that this novel project will lead to more formal interactions between the ICR and IAPH. The cover of this report features her painting Let the Skyworld Shake (2016).
Recommendations
Should the ICR Scientific Director be renewed?
Recommendation 4: The Panel enthusiastically and without reservation recommends that the ICR Scientific Director be renewed.
Future Considerations
- The Panel suggests that the SD take steps to urge CIHR to mitigate consequences arising due to loss of the cancer-specific IAB.
- The Panel also suggests that the SD explore use of the 50% carry-over in its Institute Support Grant, to better support initiatives, communication Canada-wide and replace some of the functions of the cancer IAB.
- The Panel suggests that the SD build on previously-funded STIHRs to address training gaps in priority areas of cancer research.
Other Observations
In addition to responding to the evaluation questions, there are several other observations that the Panel feels merit the attention of the Governing Council. First, it is recommended that support from 160 Elgin (CIHR headquarters) to the Institute be increased. Specifically, content experts at 160 Elgin should take responsibility for ministerial correspondence that is currently directed to the ICR, but for which the Institute is not resourced.
Next, the Panel expressed concerns that the profile of the Institute appears to be waning due, in part, to the demise of the broad-based and regionally distributed IAB. Previously, the cancer IAB served not only to provide advice to the SD, but also to communicate and network nationally with researchers, through tours and town hall meetings, and this function has been lost. The Panel proposes that resources previously devoted to convening the IAB be allocated to activities to raise the awareness of the ICR nationally within the community such as recruiting ICR ambassadors, branding initiatives, and increasing interactions with the new generation of cancer researchers through more trainee events.
IV. Evaluation Key Findings
Relevance
Ongoing Relevance of Support to ICR Research
The human and economic burden of cancer continues to be high; 2 in 5 Canadians will develop cancer in their lifetimes and 1 in 4 will die of the disease. The number of cancers is expected to increase overall due to the growing and aging population. At the same time, the scientific landscape has also evolved with novel targeted cancer therapies and companion diagnostics, harnessing basic discovery research continuing to dominate the treatment horizon; advancements in genomics, personalized medicine and emergence of promising new research in areas such as immunotherapy; survival rates are increasing for some cancers, highlighting quality of life/survivorship issues; and prevention has historically been a weak link, but increasing recognition of common risk factors, disparities.
The relevance of the mandate is largely unchanged in the last 5 years as the Institute provides a national face for cancer research, but it also fosters connections within the cancer research community and cancer research funders as a whole. Within a complex research funding environment, ICR has played a leadership role by co-chairing the CCRA which brings together 35 cancer research funders.
The ability of ICR to influence the cancer research agenda in Canada is severely limited by the amount of funding available to the Institute. The ICR’s research budget is <1% of total annual investments in cancer in Canada and approximately 1-3% of total CIHR investments annually in the ICR mandate area. Furthermore, the ICR has limited influence on the larger CIHR investigator initiate (open) grants. There also is lack of clarity in the researcher community about the limited role of the ICR in open competition CIHR funding.
Taking this into consideration, the ICR has been responsive to the broader scientific community and funding landscape. For instance, the Institute has aligned activities with other Institutes and organizations, such as the Institute of Genetics and Genome Canada to fund high caliber research in new areas. Priorities for the Institute found in the 2015-2020 Strategic Plan were based on broad consultations with the cancer research community and input from the ICR-IAB. Challenges remain as it pertains to satisfying all research areas of interest. While 68% of funded researchers say their research fits well within the ICR mandate, 19% of stakeholders say the ICR mandate allows the Institute to support their organization’s area of interest.Appropriateness of current the ICR Mandate and Changes to Institute Name
The ICR mandate is generally perceived to be appropriate and aligned with the strategic direction of CIHR overall. Given the Institute’s finite resources, the Strategic Plan and related priorities help put focus on the areas where ICR can be most impactful. There is some desire within the community to broaden the Institute’s mandate and strategic priorities. Findings showed that the current three priorities align with the interests of a smaller number of researchers/stakeholders.
With respect to the Institute’s strategic priorities, the high fatality cancer priority aligns well with the interests of many researchers and stakeholders in theme 1. The health economics/health services improvement priority has been well-received as innovative and in-line with the desire for evidence-based practices in the area, and also has the potential to provide learning examples to other Institutes. The redressing health disparities priority is viewed by some as a delivery issue more so than a research question (although the Institute is still in the process of defining this priority and developing community capacity).
A variety of potential gaps in the ICR mandate were identified by researchers and stakeholders, with predominant comments related to absence/insufficient importance to basic research. It was indicated that biomedical research remains important in advancing knowledge about cancer, but there is less emphasis currently on this area within ICR and CIHR (although it should be noted that, while the number of awards of total funding has decreased in the last two years, 65% of CIHR funding in the ICR mandate area was allocated to the biomedical theme). See Figure D in Appendix 3 for an overview of CIHR investment in the ICR mandate areas by primary theme. Additional identified gaps include: infrastructure funding, support to trainees, technology development, clinical trial activity, KT research, as well as focus on the areas of prevention, quality of care/survivorship, and global health.
There was no evidence to support the need for a change in the the Institute’s name, although some stakeholders believe the French version (Institut du cancer) requires attention as it is not consistent with the English version.
Transformative Impact
Support to Innovative Research and Advancing Knowledge
The ICR supports innovative, internationally high caliber research in areas from biomedical to the psycho-social theme. Specifically, the Institute contributes to advancing knowledge by investing in research grants in innovative areas guided by strategic priorities. This includes $103M in research grants disbursed during the 2000-01 to 2014-14 period, as well as the 13 funding opportunities launched by the ICR since 2012-13. Among the funding opportunities are: Innovation Grants; Breast Cancer in Young Women funding; and funding for a multi-disciplinary/site team in the area of cancer stem cells (along with Genome to Canada, Stand-Up to Cancer and others).
Innovative ideas in cancer research are supported to some degree directly and (even more so) indirectly when researchers leverage additional funding. Just over half (54%) of funded researchers claimed that ICR funding supported the development of innovative research ideas. One-third (33%) of ICR funded researchers stated that ICR funds helped them with securing additional funds from other organizations and 78% of those researchers indicated the funds supported the development of innovative ideas in their research. The support of innovative ideas is also seen in partnerships with stakeholders. One-third (32%) of stakeholders indicated their partnership/collaboration with the ICR supported the development of innovative ideas.
Additionally, the ICR helps to advance knowledge through sharing of findings with other researchers and trainees. Six in ten ICR funded researchers stated that ICR funding helped them share their findings with other researchers and trainees to a good extent (60% and 57% respectively). The impact study indicates that approximately 20% of the ICR mandate related publications had at least one CIHR supported author who was an Early Career Researcher at the time of publication (using CIHR’s adopted methodology for calculating career stage).
The ICR has benefited from the strong leadership of all of its SDs. The contribution of the current SD to impacts is seen in strategic investments in high impact areas, such as the new priorities of health economics/services and prevention/addressing risk factors disparities, particularly related to Indigenous health. The SD is also an effective member of the CIHR management team and is chair of CIHR’s Subcommittee on Implementation and Oversight, and collaborates with other Institutes, serving as the co-lead for the RAF-funded Personalized Medicine and Epigenetics Signature Initiatives, for example. As an active participant in the broader cancer research funding community, the SD serves as co-chair for the CCRA, and is a representative for Canada on the Governing Council for International Agency for Research on Cancer. The SD also sits on the Advisory Committee on Research for the Canadian Cancer Society and on the Scientific Advisory Committee for the Terry Fox Research Institute.
Contributions to Building Capacity of the Health Research Enterprise
Cancer research capacity in Canada is strong: there are 2000+ CIHR funded cancer researchers, 200+ directly supported trainees, and 1,300+ indirectly supported trainees are active each year in Canada in this mandate area. A significant portion of CIHR capacity building funds in each of these areas (15-20%) goes to ICR mandate areas specifically. ICR contributes to capacity building by using a variety of CIHR tools. For instance, the ICR has invested in Strategic Training Initiative in Health Research (STIHR), training, planning, catalyst/pilot and development grants, as well as training awards. Over the years ICR supported a total of 22 STIHR programs. (See Figure E, Appendix 3 for a breakdown of ICR RC investments in capacity building). Furthermore, direct and indirect capacity building occurs through funded research. For example, 60% of researchers say their ICR funding supported the training of researchers and practitioners and 82% stated this was the case with funding leveraged from other organizations.
ICR investment contributing to capacity building has decreased between 2001-02 and 2008-09 ($16M) and 2009-10 and 2014-15 ($11.5M). The ICR has been strategic in their capacity building efforts and focus has been on building the capacity of new investigators. This has occurred through annual New Investigator meetings that contribute to professional development and mentoring of junior faculty working in cancer research. The biennial Canadian Cancer Research Conference also includes an ICR-hosted session for senior traineesand early career investigators.
Contributions to Achieving Broader Health, Economic and Social Impact
Contributions to achieving broader health, economic and social impacts occur through activities the ICR undertakes to foster KT for health services improvement. It is anticipated that the new health economics/services priority will further this contribution. KT activities include ongoing researcher/knowledge user interactions through: meetings, workshops, Café Scientifique, Best Brains Exchanges, and the biennial Canadian Cancer Research Conference; a partnership with the Canadian Cancer Society Research Institute (CCSRI) to support Knowledge to Action grants; up to $1.2M planned for clinical trials in Ontario, as Stand-Up to Cancer’s Dream Team has focused on clinical deliverables; commissioning artwork depicting the cancer journey from an Indigenous Peoples’ perspective, to promote dialogue and increase awareness.
Improvements to health services and health of Canadians is a lower order impact (compared to knowledge advancement and capacity building) than advancing knowledge. As indicated by researchers and stakeholders, 26% of funded researchers stated that ICR funding supported KT toward improving Canadian health services and 27% of stakeholders say their partnership with the ICR did the same. In terms of ICR funding and partnerships supporting KT for the improvement of the health of Canadians, 29% of funded researchers and 23% of stakeholders said this was the case. Currently approximately 25% of researchers state that ICR funding helped them with knowledge sharing with other groups such as practitioners, educators, non-profits and/or policymakers.
The ICR’s capacity to further measure research impacts is limited and therefore it is difficult to attribute impact to the Institute of co-funded/co-hosted initiative. The ICR has undertaken new means to assess impacts by, for example, implementing an assets map to determine the impact of early investments in palliative cancer care research on health policy, KT, applications, and capacity building.
Convener and Catalyst
Contribution of Scientific Leadership to Convener-Catalyst Role
It is clear from surveyed stakeholders that the ICR leadership is a leading source of information in the field, is proactive in promoting the visibility of the Institute, as well as keeping stakeholders informed. While the Institute’s leadership and active involvement in the community is strong, there are mixed views regarding the awareness and clarity of the Institute’s role within the research community. The profile of the Institute and its work were generally perceived to be low by key informants who also noted confusion within the cancer research community about open and priority-driven funding opportunities and the role of the Institute.
Key vehicles for engagement in recent years included: 1) the CCRA platform for knowledge sharing and collaboration; 2) consultations during the development of 2015-2020 Strategic Plan; and 3) the co-hosting of the biennial Canadian Cancer Research Conference. Broader researcher and stakeholder community engagement with the ICR occurs through the ICR newsletter.
Partnering to Achieve CIHR and Institute Objective
The evidence suggested that ICR has been an effective convener and catalyst. The evaluation identified several benefits to the ICR’s partnerships and collaborations with other entities such as varied input on priorities/multi-dimensional perspectives to complex problems, coordination of shared research interest, broader awareness and participation in co-hosted events, efficiency/leveraging of infrastructure, and credibility for charities with donors.
A key success of the Institute has been the creation and fostering of partnerships with other organizations. These partnerships have leveraged significant and increasing funds toward CIHR and Institute priorities. In total, ICR leveraged $80M in contributions from 52 partners between 2000-01 and 2014-15 (one-third from Genome Canada). The annual average partner leveraging ratio increased from 23% between 2002-03 and 2009-10 to 149% between 2011-12 and 2014-15. One initiative – the partnership with Stand Up to Cancer – had an ICR investment of $1.5M which led to a total $11.7M fund for a Dream Team research initiative on brain cancer. The leveraging ratio is significantly higher for the ICR compared to overall CIHR funding in the ICR mandate area, for example 130% for ICR compared to 15% for CIHR overall 2014-15.
The Institute and its leadership are very active in CIHR Signature Initiatives, as well as co-funded/co-sponsored funding opportunities and events with other cancer organizations. The ICR is partner in 6 Signature Initiatives and co-lead for two others (i.e. Personalized Medicine and CEEHRC). (See Figure F, Appendix 3 for ICR investments in Major Initiatives between 2011-12 and 2014-15.)
Other ICR partnered activities include: CCSRI, Genome Canada, Canadian Breast Cancer Foundation, Stand Up to Cancer, and Canadian Partnership Against Cancer.
Operational Effectiveness
ICR Effectiveness
Evidence indicated that the ICR, as an entity, is operating effectively. In addition to ICR’s research funding opportunities (see Figure G in Appendix 3), the Institute works to influence through other no- or low-cost initiatives such as workshops and meetings. The implementation of ICR’s current Strategic Plan is occurring in phases and geared to resources available, moving sequentially to address the three priority areas: most advanced on Targeting High Fatality Cancers, then Health Economics and Health Services Research in Cancer Control, followed by Redressing Cancer Risk Factor Disparities and Prevention Service Inequities.
The ICR, as with all CIHR Institutes, receives $1M annually through the Institute Support Grant (ISG) for operating and development expenditures, which is separate and distinct from the ICR’s annual research budget. Each year the unspent balance of the ISG is carried over to the next fiscal year, hence the total annual funds available in this category can exceed the $1M annual allotment to the Institute. Over the period from 2009-10 to 2015-16, the ICR typically spent its full annual allotment of $1M, which represented approximately 50%-60% of funds available under the ISG.
The reforms at CIHR impacting the Institutes – the Institutes’ contributions to the CIHR's RAF, restructuring of corporate support – have not led to any significant negative impacts on ICR. Participation in the RAF continues the ICR’s pre-existing active involvement in multi-Institute initiatives and the CIHR extended virtual team is noted to be providing adequate support. However, restructuring of the IAB has created a need to seek alternative means to gather cancer-specific guidance and to ensure corporate memory of the Institute during SD transition.
V. References
Canadian Cancer Society. (2015). Canadian Cancer statistics. Retrieved from Special topic: Predictions of the future burden of cancer in Canada
Canadian Cancer Statistics. 2016. Canadian Cancer Society.
Cancer Res; 74(11); 2913–21; 2014 AACR.
Canadian Cancer Research Alliance. (2015). Cancer Research Investment In Canada, 2013.
Retrieved from Canadian Cancer Research Alliance annual CIHR. The ICR Useful Links. (2015). Retrieved from Institute of Cancer Research.
IARC. (2012). All cancers excluding non-melanoma skin cancer. Estimated incidence, mortality and prevalence. The ICR’s SD continues to be active partners within this organization (i.e. current SD Dr. is the CCRA Co-chair).
Public Health Agency of Canada 2014. Economic Burden of Illness in Canada, 2005–2008. Ottawa.
Statistics Canada, (2011). Leading causes of deaths in Canada, 2011. CANSIM table 102-0522.
VI. Appendices
Appendix 1 : The ICR Evaluation Panel Members’ Affiliations and Conflict of Interest Declaration
Chair
- Ann Chambers, Professor, Department of Oncology, Schulich School of Medicine and Dentistry, University of Western Ontario
Panel Members
- Pamela Hoodless, Professor, Medical Genetics, University of British Columbia
- Roger Deeley, Vice-Dean, Health Sciences Research, Queen's University
- Ken Kao, Professor, Division of Biomedical Science, Faculty of Medicine, Memorial University
At the outset of the ICR Evaluation Panel Synthesis Workshop in February 2017, all Panel members were invited to declare any conflict of interest that could impair their ability to perform their duties as ICR Evaluation Panel members in an objective and impartial manner.
| Panel Member | Conflict of Interest Declaration |
|---|---|
| Ann Chambers | Confirmed no real, apparent or potential conflict(s) of interest with respect to her involvement with the Evaluation Panel |
| Pamela Hoodless | Confirmed no real, apparent or potential conflict(s) of interest with respect to her involvement with the Evaluation Panel |
| Roger Deeley | Confirmed no real, apparent or potential conflict(s) of interest with respect to his involvement with the Evaluation Panel |
| Ken Kao | Confirmed no real, apparent or potential conflict(s) of interest with respect to his involvement with the Evaluation Panel |
Appendix 2 : Overview of Data Sources and Methods
| Data Source | Description | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Situational analysis |
|
||||||||||||||||||
| Impact study on ICR related research within and beyond academia |
|
||||||||||||||||||
| Key informant interviews |
|
||||||||||||||||||
| Researcher survey |
|
||||||||||||||||||
| Stakeholder survey |
|
Note: These data sources were complemented by telephone consultations, conducted by the ICR Evaluation Panel during the two-day face-to-face meeting, with six key members of the ICR research community who had not been previously interviewed (although some may have completed the researcher survey).
Appendix 3 : Key Figures and Tables
Figure A: Proportion of death due to cancer and other causes, Canada, 2011
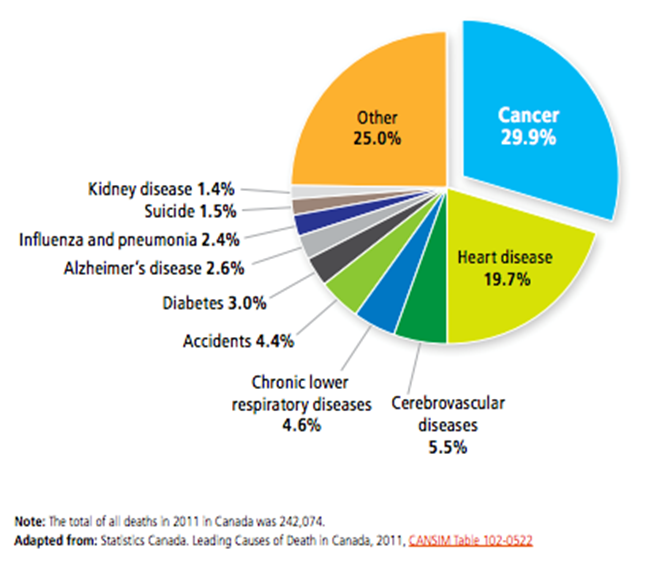
Figure A long description
| Deaths due to cancer and other causes | Proportions |
|---|---|
| Cancer | 29.9% |
| Heart disease | 19.7% |
| Cerebrovascular diseases | 5.5% |
| Chronic lower respiratory diseases | 4.6% |
| Accidents | 4.4% |
| Diabetes | 3.0% |
| Alzheimer disease | 2.6% |
| Influenza abd pneumonia | 2.4% |
| Suicide | 1.5% |
| Kidney disease | 1.4% |
| Other | 25% |
Figure B: Percentage of ICR RC Investment out of CIHR Investment in ICR mandate
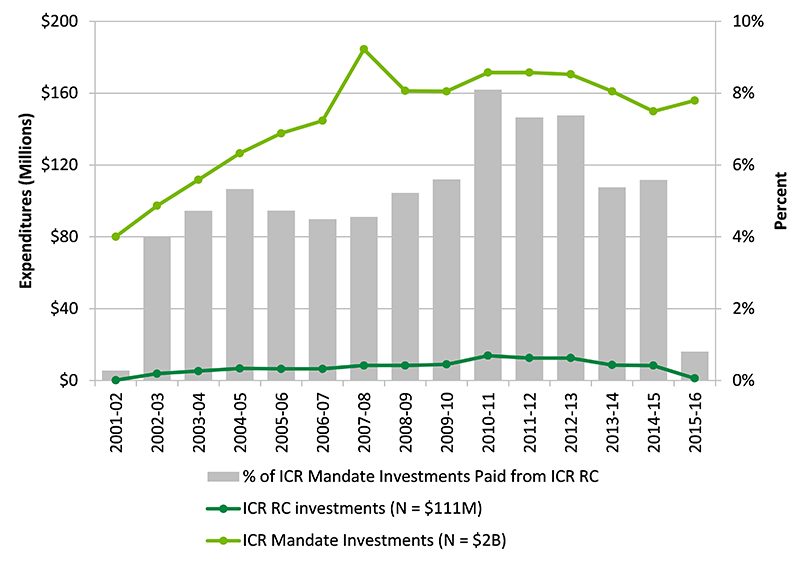
Figure B long description
| 2001-02 | 2002-03 | 2003-04 | 2004-05 | 2005-06 | 2006-07 | 2007-08 | 2008-09 | 2009-10 | 2010-11 | 2011-12 | 2012-13 | 2013-14 | 2014-15 | 2015-16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICR RC investments (N = $111M) | $222,518 | $3,895,087 | $5,292,158 | $6,751,931 | $6,520,244 | $6,507,315 | $8,404,704 | $8,438,026 | $9,017,160 | $13,894,070 | $12,567,472 | $12,588,969 | $8,665,873 | $8,379,809 | 1265963 |
| ICR Mandate Investments (N = $2B) | $80,138,464 | $97,387,259 | $111,858,657 | $126,577,191 | $137,672,077 | $144,724,001 | $184,490,395 | $161,330,701 | $161,038,286 | $171,556,037 | $171,521,059 | $170,515,331 | $161,042,655 | $149,922,615 | 155976396.3 |
| % of ICR Mandate Investments Paid from ICR RC | 0% | 4% | 5% | 5% | 5% | 4% | 5% | 5% | 6% | 8% | 7% | 7% | 5% | 6% | 1% |
Figure C: Partners’ Contributions to ICR Priorities, ICR RC Investments and Leverage Ratio of Partnership from 2001-02 to 2015-16
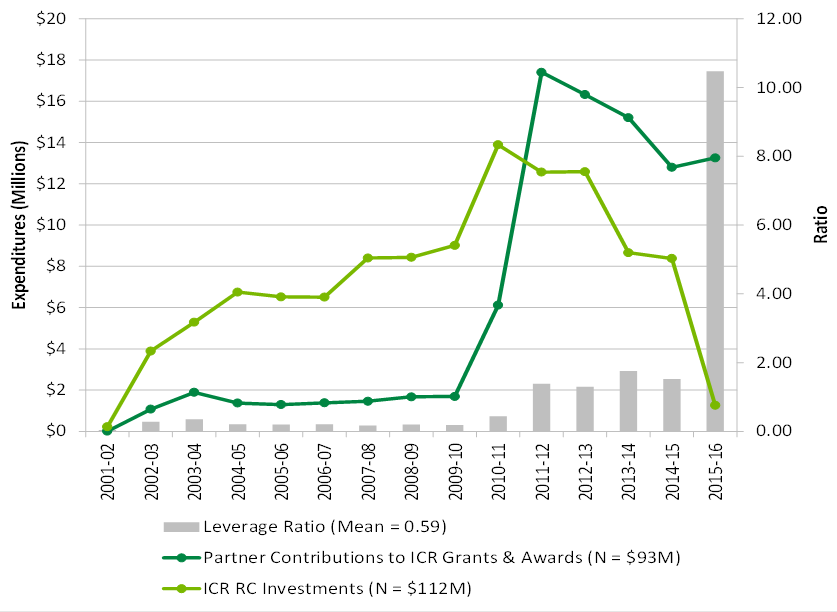
Figure C long description
| 2001-02 | 2002-03 | 2003-04 | 2004-05 | 2005-06 | 2006-07 | 2007-08 | 2008-09 | 2009-10 | 2010-11 | 2011-12 | 2012-13 | 2013-14 | 2014-15 | 2015-16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Partner Contributions to ICR Grants & Awards (N = $93M) | $9,999 | $1,079,500 | $1,892,750 | $1,373,286 | $1,301,476 | $1,385,780 | $1,461,652 | $1,678,750 | $1,692,720 | $6,116,196 | $17,407,453 | $16,322,035 | $15,207,184 | $12,799,314 | $13,257,066 |
| ICR RC Investments (N = $112M) | $222,518 | $3,895,087 | $5,292,158 | $6,751,931 | $6,520,244 | $6,507,315 | $8,404,704 | $8,438,026 | $9,017,160 | $13,894,070 | $12,567,472 | $12,588,969 | $8,665,873 | $8,379,809 | $1,265,963 |
| Leverage Ratio (Mean = 0.59) | 0.04 | 0.28 | 0.36 | 0.20 | 0.20 | 0.21 | 0.17 | 0.20 | 0.19 | 0.44 | 1.39 | 1.30 | 1.75 | 1.53 | 10.47 |
Figure D: CIHR Investment in the ICR mandate areas by Primary Theme
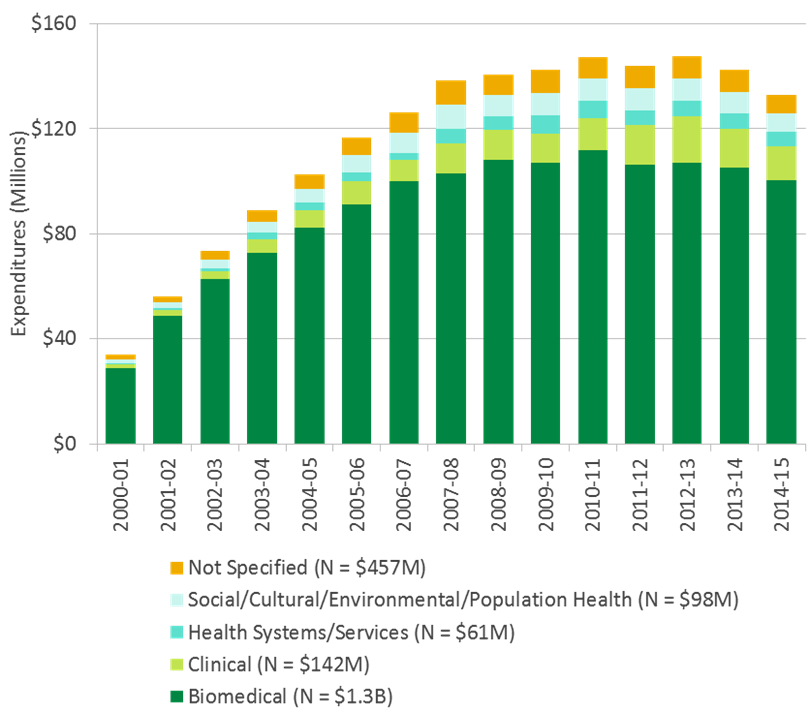
Figure D long description
| total | 2000-01 | 2001-02 | 2002-03 | 2003-04 | 2004-05 | 2005-06 | 2006-07 | 2007-08 | 2008-09 | 2009-10 | 2010-11 | 2011-12 | 2012-13 | 2013-14 | 2014-15 | 2015-16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomedical (N = $1.4B) | 1,440,893,178 | 28,649,990 | 48,842,901 | 62,606,285 | 72,834,199 | 82,205,795 | 91,003,804 | 99,969,990 | 102,974,260 | 108,202,948 | 106,871,130 | 111,670,969 | 106,239,747 | 106,865,994 | 105,207,051 | 100,419,886 | 106,328,230 |
| Clinical (N = $153M) | 153,828,913 | 1,577,487 | 2,103,460 | 2,984,287 | 5,068,248 | 6,625,366 | 8,928,382 | 8,265,388 | 11,343,508 | 11,371,560 | 11,330,415 | 12,125,158 | 14,977,741 | 17,776,759 | 14,611,570 | 12,920,188 | 11,819,397 |
| Health Systems/Services (N = $65M) | 65,386,020 | 355,786 | 599,254 | 1,272,732 | 2,471,287 | 2,969,760 | 3,394,035 | 2,553,753 | 5,504,878 | 5,197,391 | 6,751,770 | 6,740,741 | 5,723,188 | 6,049,411 | 6,023,865 | 5,306,066 | 4,472,103 |
| Not Specified (N = $482M) | 481,956,993 | 29,458,520 | 26,378,482 | 27,286,059 | 27,196,162 | 29,436,444 | 27,738,001 | 26,266,347 | 55,482,930 | 28,667,124 | 27,340,989 | 32,604,822 | 36,158,485 | 31,311,830 | 26,960,650 | 24,220,793 | 25,449,356 |
| Social/Cultural/Environmental/Population Health (N = $105M) | 105,372,499 | 1,644,697 | 2,214,367 | 3,237,897 | 4,288,760 | 5,339,825 | 6,607,856 | 7,668,522 | 9,184,821 | 7,891,678 | 8,743,982 | 8,414,348 | 8,421,898 | 8,511,337 | 8,239,519 | 7,055,682 | 7,907,311 |
| Grand Total | 2,247,437,603 | 61,686,480 | 80,138,464 | 97,387,259 | 111,858,657 | 126,577,191 | 137,672,077 | 144,724,001 | 184,490,395 | 161,330,701 | 161,038,286 | 171,556,037 | 171,521,059 | 170,515,331 | 161,042,655 | 149,922,615 | 155,976,396 |
Figure E: The ICR RC Investment in Capacity Building
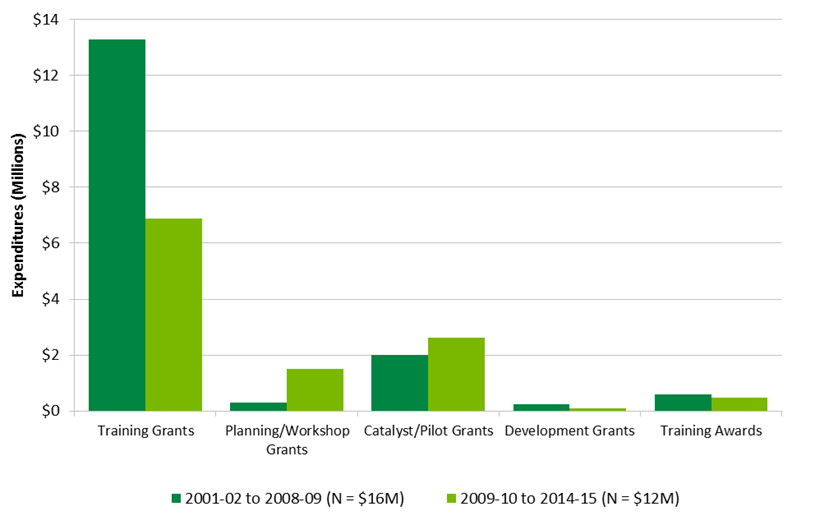
Figure E long description
| 2001-02 to 2008-09 (N = $16M) | 2009-10 to 2015-16 (N = $12M) | |
|---|---|---|
| Training Grants | $13,278,874 | $6,870,222 |
| Planning/Workshop Grants | $282,796 | $1,603,892 |
| Catalyst/Pilot Grants | $2,006,560 | $2,663,204 |
| Development Grants | $238,936 | $104,138 |
| Training Awards | $579,414 | $588,295 |
Figure F: Cumulative Institute Investments in Major Initiatives between 2011-12 and 2014-15
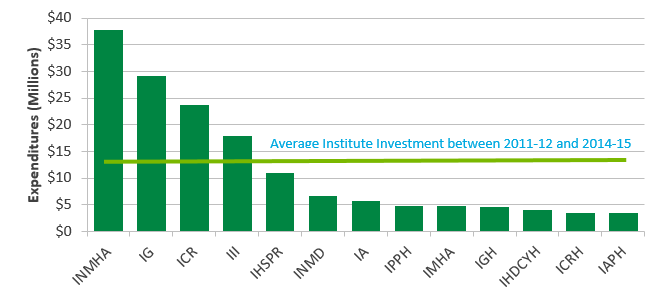
Figure F long description
| total | 2011-12 | 2012-13 | 2013-14 | 2014-15 | |
|---|---|---|---|---|---|
| INMHA | $37,689,381 | $6,611,733 | $7,583,715 | $11,073,218 | $12,420,715 |
| IG | $29,131,506 | $5,361,459 | $5,942,484 | $8,132,630 | $9,694,933 |
| ICR | $23,691,262 | $4,823,516 | $7,272,174 | $5,394,655 | $6,200,917 |
| III | $17,916,997 | $3,009,555 | $2,983,862 | $6,038,094 | $5,885,486 |
| IHSPR | $10,951,696 | $1,003,056 | $2,649,424 | $3,770,572 | $3,528,644 |
| INMD | $6,576,034 | $795,637 | $1,086,671 | $2,186,290 | $2,507,436 |
| IA | $5,678,284 | $731,658 | $283,852 | $1,934,343 | $2,728,431 |
| IPPH | $4,879,406 | $129,164 | $550,000 | $1,934,942 | $2,265,300 |
| IMHA | $4,778,567 | $615,086 | $849,965 | $1,240,978 | $2,072,538 |
| IGH | $4,563,962 | $264,306 | $450,000 | $1,675,000 | $2,174,656 |
| IHDCYH | $3,981,590 | $337,500 | $369,090 | $1,475,000 | $1,800,000 |
| ICRH | $3,445,216 | $605,494 | $253,214 | $1,361,508 | $1,225,000 |
| IAPH | $3,409,667 | $5,767 | $178,806 | $1,436,143 | $1,788,951 |
| $12,053,351 |
Cumulative Institute Investments in Major Initiatives between 2011-12 and 2014-15
Figure G: The ICR Current Funding Opportunities
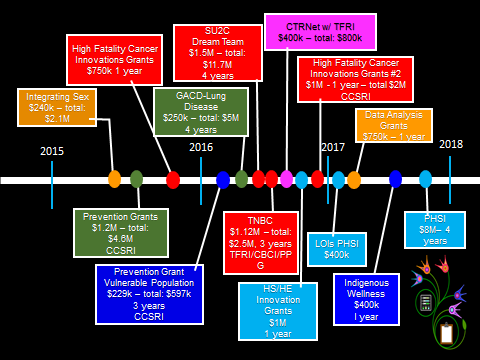
Figure G long description
| ICR Funding opportunity | Total funds | Amount per grant | Grant duration |
|---|---|---|---|
| Integrating Sex | 2.1 M | 240K | 1 year |
| Prevention Grants | 4.6M | 1.2M | 4 years |
| High Fatality cancer Innovation Grants | 750K | Up to 100K | 1 year |
| Prevention Grant - Vulnerable populations | 597K | 229K | 3 years |
| GACD- Lung Disease | 5M | 250K | 4 years |
| SU2C Dream Team | 11.7M | 1.5M | 4 years |
| Triple Negative Breast Cancer (TNBC) | 2.5M | 1.12 M | 3 years |
| Canadian Tumour Repository Network with Terry Fox Research Institute (CTRNet w/TFRI ) | 800K | 400K (how much ICR contributed) | 4 years |
| Innovation Grants - Health Services/Health Economics (HS/HE) | 1M | 100K | 1 year |
| High Fatality Cancer Innovation Grants #2 | 2M | 1M | 1 year |
| Letter of intent (LOIs) Partnerships for Health Services Improvement (PHSI) | 400K | 20K | 1 year |
| Data Analysis Grants | 750K | 75K | 1 year |
| Indigenous Wellness | 400K | 150K | 1 year |
| Partnerships for Health Services Improvement (PHSI) | 8M | 1M | 4 years |
- Date modified: