Sequencing Our Future: Institute of Genetics Strategic Plan 2022-2027
Commitment A: Enabling Genomic Medicine
Commitment A: Enabling Genomic Medicine

Genomic medicine is at the cusp of a learning health system wherein patient information informs research, which in turn drives clinical care. This genomic learning health system will increasingly accurately determine what within our genomes keeps us healthy, and what results in sickness or predisposition to disease. Using genomics to stratify inherited and acquired diseases is already a reality. Expanding the number of people living in Canada whose genomes are sequenced and analyzed will result in an increasingly impactful effect of genomics on healthcare.
A unified strategy to generate store, access, and share genomic information is a necessary and defining step to enable genomics to improve the health for people who live in Canada.
Early Steps
An essential early step for such a strategy is the creation of a single-entry point to all genomes sequenced in Canada. A governance structure will establish a common set of standards to ensure interoperability, manage access to data, and address ethical and legal aspects while managing the data infrastructure and computing power. Early engagement of the public and patients on issues such as privacy and security will be crucial to its success. This engagement will need to include research centered on the patient/community needs as well as their participation in all stages of studies, from co-design to execution to implementation.
Why genomics and why now? Simply put, the time and cost of sequencing a human genome has decreased over time, from taking over a decade to complete the first working draft of the human genome in 2001, to less than a day in 2021. The cost decreased from over $1 billion in 2001, to $50,000 in 2010, to $750 currently, and is still decreasing. Genomics research is increasingly being integrated into clinical care and is starting to be incorporated into medical records (personalized medicine). To further put this advance into perspective, a few hundred thousand human genomes have been sequenced worldwide thus far, and this will increase exponentially such that over 60 million human genomes are anticipated to be sequenced by 2026.
Canadian Human Genome Library – a national asset
A responsibly governed Canadian Human Genome Library is a key research and clinical resource that will be designed to ensure that human genome sequencing efforts can unite in real time to enable a learning health system. This will also enable Canada to be better prepared to answer the challenges brought by any future viral or multi-drug-resistant bacteria outbreaks. The development of this library will represent a structuring initiative and will be empowered by combining strengths from the Health, and the Innovation, Science and Economic Development Portfolios.
Towards Predictive and Personalized Medicine
Genomics Research to Health Care Infinity Loop
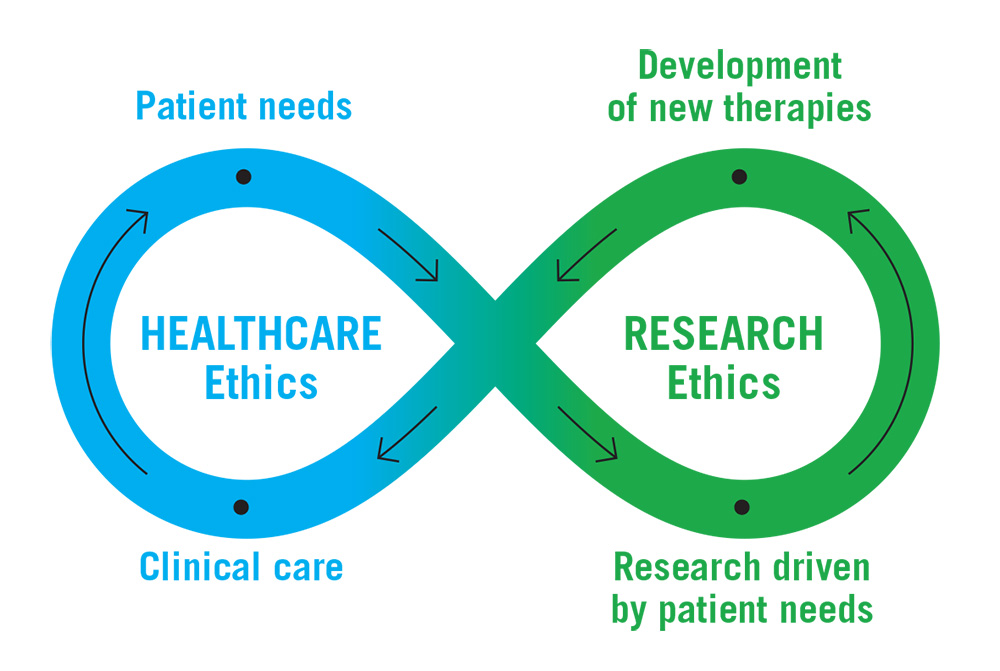
Long description
Infinity loop with arrows directing the continuous flow. On the left side is Healthcare Ethics with points on the loop labelled "Patient needs" and "Clinical care". On the right side is Research Ethics with points on the loop labelled "Development of new therapies" and "Research driven by patient needs".
"Patient needs" flows into "Research driven by patient needs", which flows into "Development of new therapies", which then flows into "Clinical care" and then back into "Patient needs".
One major advantage of genomes versus many other tests is that a person’s genome is normally static: it does not change over their lifespan. It is a test that is only done once (unlike blood pressure, cholesterol levels, heart/brain/liver function, etc.) and can be analyzed and reanalyzed over a person’s lifespan. Our knowledge of what is within our genomes that keeps us healthy and makes us sick is becoming increasingly accurate. This new knowledge can be integrated throughout a person’s life, including reanalysis of genomes, health records, and other data sources, thereby increasing the value of a learning health system.
As your once-in-a-lifetime genome sequence becomes increasingly incorporated into healthcare data and its analysis, this will enable a major goal of genomic medicine: preventing disease before its onset. As more genomic data sets become available, polygenic risk scores will enable predictions for the potential for common diseases (e.g. heart disease and stroke, cancer, diabetes, and many others) to be made at an individual level. Ever-expanding genomics and health data sets will enable Artificial Intelligence (AI) to be used to determine what combination of genes predisposes a person toward a specific disease(s). As other omics technologies come online (that can indicate acute changes in levels of proteins, metabolites, and other biological reporters) even more accurate predictors of disease risk will be enabled. This information can guide more preventative medicine, resulting in delay or prevention of the onset of a chronic disease or of the occurrence of an acute event (e.g. heart attack, stroke, or diabetes, cancer, mental illness, etc.)
Establishing accurate polygenic risk scores to improve risk prediction can be used for common diseases. These can be incorporated into the clinic in ways that will decrease healthcare burden and increase quality of life for the people who live in Canada. As this knowledge changes healthcare, these advancements need to be instituted in a manner that involves patients and the public in its implementation. This demands clarity in how data will be employed, meeting necessary ethical, legal, and regulatory requirements to ensure public trust. The infinity loop driving the genomic learning health system will be an example of the responsible use of health data for the benefit of patients, and for society at large.
Preventing Adverse Events
Beyond disease prediction, genomics can improve drug safety. Genetics can predict if a particular drug will be effective and/or cause harm to a particular patient. Adverse drug reactions to medicines account for ~10% of Canadian hospital admissions with an average stay of one week. Cross-referencing genomics with prescribing of medicines (known as pharmacogenomics) can decrease adverse events, decrease hospital admissions and associated costs, and ensure only patients that are most likely to benefit from a therapy will receive it.
Response to Emerging Health Threats
Genomics extends beyond human genomes to other organisms, the most relevant currently being the SARS-CoV-2 coronavirus that causes COVID-19. The critical role of genomics in the COVID-19 response is the sequencing of viral genomes in patients. Viral genome sequencing also enables tracking of the global and national spread of COVID-19 variants and helps determine which of those is of concern; essentially which versions of the virus are more virulent and/or more deadly. Tracking emerging variants of concern imported into Canada is an essential part of the contact tracing to detect, and thus decrease the spread of these more virulent strains.
In addition, genome sequencing of the SARS-CoV-2 coronavirus in infected patients is essential to rapidly identify and characterize any potential ‘made in Canada’ variants of concern. Once identified, variants of concern can then be analyzed to determine how they spread more quickly, why they are more deadly, or if they can evade current vaccines.
On the human (host) side, there are varied responses to COVID-19 infection, even among healthy people infected with the same COVID-19 strain. Some people have mild to no symptoms, while others die. Sequencing the genomes of infected patients will increase our understanding of the biology that results in differences in disease severity among individuals. These could range from the ability of COVID-19 to infect cells, to its ability to replicate and spread within a person’s body, to its ability to initiate a cytokine storm and lead to more severe disease or death, or to the development of long COVID in some patients. To maximize the impact of genomics in the international efforts against COVID-19, there is a pressing need to streamline, accelerate and facilitate data sharing of genomic and associated clinical and large metadata sets, both at the national and at the international levels.
International Genomic Data Sharing
Enabling genomics in medicine requires the sharing of large and diverse data sets, validated tools for data quality, access, and analysis, and high-end computational power. Over the past decade, the Global Alliance for Genomics in Health (GA4GH) has emerged as the international leader to establish standards and a common framework to enable large-scale international genomic and healthcare data sharing. These past efforts have been a major driver behind the global response to sharing of COVID-19 variant genomes by many nations, including Canada. Rapid sharing of high-quality data is critical for the scientific understanding that underpins an effective and timely response to any pandemic.
Now officially established in Canada, GA4GH continues to develop and promote the creation of real-world genomic data initiatives. This is done through the adoption of tools and standards for sharing of genomic data, including regulatory and ethical aspects. Among others, they address issues pertaining to the return of results to patients, data security, data access, and data sharing.
The GA4GH framework for responsible sharing of data is based on the idea that science and society are partners in determining data sharing principles. This framework is founded on the premise that everyone benefits from science and its applications as stated in the Universal Declaration for Human Rights, 1948, Article 27. Now being internationally recognized as a core resource, international data projects are incorporating GA4GH standards and policies.
The CIHR Institute of Genetics is committed to ensuring GA4GH standards and principles will be used to enable national and international data sharing to advance the capacity of genomics to increase the health and welfare of the people who live in Canada as well as all peoples worldwide. This aligns with Canada’s Research Data Management Framework which is aiming to enable open science and data sharing. Indeed, to ensure an equitable access and participation in both the scientific process and its benefits, Canada must rigorously maintain technical and ethical standards that support the open sharing of data and knowledge – now and always.
Enabling Genomic Medicine – Supporting CIHR’s Priorities
Our commitment towards the development and implementation of genomic medicine aligns with and supports all five of CIHR’s priorities. Through the promotion of open science, implementation of FAIR principles, and the support of national cross-cutting initiatives and international collaboration, this initiative is all about “advancing research excellence in all of its diversity” (Priority A). By providing an essential structure to responses to emerging health threats, it will also “strengthen Canadian health research capacity” (Priority B). By creating an ecosystem that supports Indigenous (First Nations, Métis, Inuit) self-determination and governance, this commitment aims to reduce the “genomic divide” and improve the well-being of Indigenous people (Priority C). Also, by strongly supporting the identification of determinants of health, this commitment will pave the way to more predictive medicine (Priority D). Ultimately, the core of this commitment is to contribute to the establishment of a true learning health system (Priority E).
- Date modified: