CIHR Pan-Canadian Network for HIV/AIDS and STBBI Clinical Trials Research Funding Opportunity
The following schema is related to the CIHR Pan-Canadian Network for HIV/AIDS and STBBI Clinical Trials Research funding opportunity.
Long description
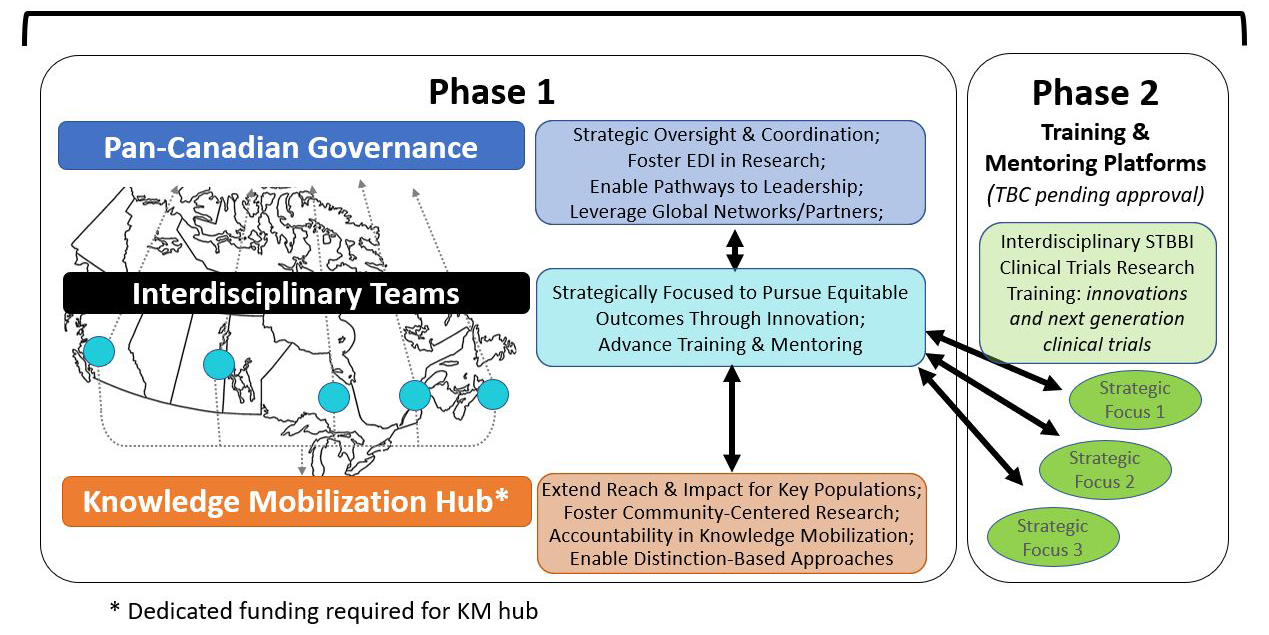
The Network funding opportunity will include two phases. The Phase 1 funding opportunity structure is designed to include pan-Canadian Governance, Interdisciplinary Clinical Trial Research Teams, and a Community-Centred Knowledge Mobilization (KM) Hub. Phase 2 will provide funding to develop multiple platforms for interdisciplinary training and mentoring that will foster the approaches and skills necessary to address the emerging needs of clinical trials research for STBBI and related conditions. It is expected that the Network will have a pan-Canadian governance structure to provide collective leadership, strategic oversight and a coordinating function for the regionally distributed Teams, the KM Hub and future Training and Mentoring platforms to be established in the Phase 2 funding opportunity. The Network governance will foster EDI in research, enable pathways to leadership for early- and mid-career researchers, and leverage demonstrate global networks and partners to optimize reach and impact. The Network must include a minimum of five (5) Interdisciplinary Clinical Trials Research Teams from different provinces and/or territories, each with representation in governance and the KM Hub to provide equitably distributed national research infrastructure. The Interdisciplinary Teams will be strategically focused to pursue equitable outcomes through innovation and will be responsible for advancing training and mentoring within the Network. The KM Hub will extend the reach and impact of the Network for key populations, foster community-centred research, provide accountability in knowledge mobilization and enable distinction-based approaches to be incorporated in Network activities and research. The Phase 2 funding opportunity, which will be launched pending approval, will support training and mentoring platforms and will include funding for multiple interdisciplinary STBBI clinical trials research training platforms connecting to the strategic priorities of the teams. The training and mentoring platforms are intended to support innovations and support the next generation of clinical trials.
- Date modified: